 |
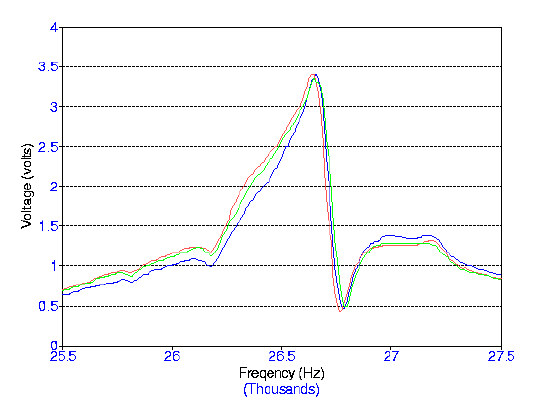
Frequency Range: 25,500 - 27,500 Hz Volume Range: 128 mL Input Voltage: 7.00 Volts Blue: Tuesday, 9 July 2001 Red: Wednesday, 10 July 2001 Green: Thursday, 11 July 2001
|
Single-Bubble Sonoluminescence
In the beginning, Gaitan said
Sonoluminescence is the process where a bubble of gas (usually air) is
created and forced to be suspended at a specific frequency inside a
flask full of a certain liquid (usually water). The bubble
experiences vibrations and is forced to rapid continuous contractions
that enables the bubble to emit flashes of light. In other words,
creating flashes of light from sound waves.
The flashes of light from the bubble are emitted with precise
regularity in the picosecond scale (10-12 seconds) with
basic laboratory equipment. This makes sonoluminescence one of the
most inexpensive way to create flashes of light of that size. In
addition, the size of the sonoluminescent bubble is so small and the
flashes of light are so brief that in order to be measured very
precise equipment needs to be used. Even with this precise equipment,
only estimates have been obtained.2
During World War I, chemists in Germany working with sonar
systems were able to initiate a chemical reaction in a liquid
solution. Reinhard Mecke from the University of Heildelberg then
suggested that the energy needed to initiate this chemical reaction
was the same as the energy needed to excite the emission of light from
an atom.3
In 1934, H. Frenzel and H. Schultes 4 from the University
of Cologne were the first to observe (multi-bubble) sonoluminescence
while conducting research in marine acoustic radars by exposing
photographic plates using acoustic sound waves to create cavitation.
The process of cavitation is one where bubbles of gas are able to
expand and contract due to changes in pressure. Their final
explanation of sonoluminescence was that the emission of light was due
to frictional energy. Frenzel and Schultes ended their paper
commenting that they had other important things to work
on.2,3
Over the years numerous researchers tried to continue the study of
multi-bubble sonoluminescence. Most of the research was inconsistent
and stagnant. The majority of the studies that were able to produce
sonoluminescence were just able to produce numerous amounts of bubbles
that expanded, contracted, collapsed, and emitted light randomly,
unpredictably, and chaotic.2,3
It wasn't until 1988 that D.F. Gaitan and L.A. Crum at the University
of Mississippi were successful in isolating a single sonoluminescent
bubble of air at a center of a flask using acoustic
resonances.2 Single-bubble sonoluminescence was born.
After a few years of experiments here and there they eventually
abandoned that field of research. Eventually the work was taken up by
S.J. Putterman and B.P Barber at UCLA, where Putterman is now one of
the most knowledgeable people in sonoluminescence.3
The sonoluminescent bubble experiences continuous rapid contractions
and expansions with precise regularity, a process that has been
extremely studied by physicists. After numerous studies, researchers
estimate that a single flash of light lasts about 50 picoseconds, with
a time between flashes to be about 35 microseconds, a relation that has
not been able to be explained with theories of bubble
dynamics.2,3 As mentioned earlier, very precise equipment
has been used to measure pulse durations. Many have used
photomultipliers amongst many things with incredibly fast response
times, but only to be able to measure the impulse response times of
photomultiplier tubes.2
The size of the bubble can be measured by Mie-scattering, shooting a
laser beam and measuring the amount of light scattered from the laser
beam. This can be measured by looking at the intensity of the
scattered laser beam because it is dependent on the square radius of
the sonoluminescent bubble. The square root of the amplitude measured
will therefore yield the radius of the bubble.3
The bubble is first the size of a few microns (micrometers,
10-6 meters). The resonant sound waves then allow the
flask to experience pressure changes. These pressure changes put the
liquid used inside the flask under tension and the size of the bubble
increases to about 50 microns. The volume of the bubble increases and
the amount of atoms and molecules inside will remain the same. With
this reasoning, the bubble will reach its maximum size and the inside
of the bubble will be close to being a vacuum. The inside and the
outside of the bubble at this point differ in pressure, the outside of
the bubble being subjected to atmospheric pressure at all times. This
difference in pressures will lead to a collapse of the bubble. The
size will then decrease to about 0.5 micron. This size will be the
smallest that it can get due to repulsion of atoms and molecules.
Therefore, one can say that the size of the bubble is dependent on the
gas that is trapped inside of it.3
In addition, the size of the bubble is also dependent on the gas
dissolved at the surface of the water. Some of this gas that has been
dissolved at the surface will eventually get into the bubble. This
gas will diffuse into the bubble when the bubble is greater in size
due to the low pressure inside. The gas will diffuse out of the
bubble when the bubble is smaller in size due to the high pressure
inside. This average of the two flows will ultimately determine the
size of the bubble.2,3
Unfortunately, there is a discontinuity with high resonant sound
waves. As the amplitude of the resonant frequency is increased, the
average size should increase along with it. Experimentally this does
not occur at higher amplitudes. There is a change before the flashes
of light and the size of the bubble becomes smaller for a little
while. After the emission of light, the bubble then continues
increasing as the amplitude of the resonant sound waves is
increased.3
Another feature of sonoluminescence is the amplitude in which the
resonant frequency is located. The sonoluminescent bubble must be
driven in an acoustic field where the bubble will be able to expand
and contract. If the field is too low, the bubble will be unable be
sustained in the flask and undergoes radial pulsations. As the field
increases, the sonoluminescent bubble will then relocate itself
between the nodal and anti-nodal regions of the flask. If the field is
too high, the bubble will be unstable and will dissipate.2
The collapse of the bubble is where the luminescence comes in. This
occurs as the bubble slows the contraction process right before it
gets to its smallest size. The theory that has been mostly agreed
upon says that up to this point most of what it is going on is due to
adiabatic heating (heating process where no heat is transfered from
and to the system). The compression of gas then allows the internal
energy to increase due to the work done during the process, which
leads to an increase in temperature. Therefore, for a sonoluminescent
bubble, where there is no heat transfer, the volume decreases, the
temperature rises, and the bubble is able to sustain higher pressures.
In addition, the amplification of this phenomenon is made by the
spherical shock wave that it is created within the bubble as it
changes. Light is emitted. The emission of light then causes the
bubble to increase in size just a little bit. The process is then
repeated again and again.3
Others believe that the light created by sonoluminescence is solely
created by the shock wave. The sonoluminescent bubble is symmetric,
forced to remain spherical due to small amounts of surface tension
until its explosion. Using this theory, temperatures inside the
sonoluminescent bubble can be expected to reach 108
Kelvin.2
A sonoluminescent bubble gives off light that is for the most part
ultraviolet. By theory, these rapid contractions and expansions allow
the bubble to sustain temperatures much higher than the surface of the
sun. In 1991, a measurement by R.A. Hiller under the supervision of
Putterman was able to measure photon energies of 6 eV, which
corresponds to a temperature of inside the bubble of about 72,000 K.
These temperatures are achieved due to the rapid collapse, which in
where a spherical shock wave just mentioned is produced. One
conclusion about the temperatures is that the surface of the bubble
does not evaporate due to the rapid changes in heating and
pressure.3
Sonoluminescence is also a highly sensitive phenomenon. It has been
recorded that sonoluminescence is also dependent on temperature of the
water inside the flask. Putterman even states that the amount of
light that a sonoluminescent bubble gives off increases 200 times when
the temperature decreases from 35 degrees Celsius to 0 degrees Celsius.
3
Many people have also tried to find sonoluminescence using different
liquids. For the most part these type of experiments have been
unsuccessful, even though there is no explanation on why other liquids
should not work. The other mixture besides water that has also been
used where people actually were able to produce sonoluminescence is a
mixture of water and glycerene. In addition, other gases besides air
(78% nitrogen, 21% oxygen, and traces of Argon, water, and carbon
dioxide amongst many others) has been used to create a bubble of air.
Surprisingly, using a mixture of liquid air (80% nitrogen and 20%
oxygen) yields a low intensity of sonoluminescence. The addition of
1% argon increases the intensity almost back to normal. The addition
of any other noble gas (helium, xenon) also brings about
sonoluminescence, each one with a unique spectrum. But overall, the
role of these inert gases is still unknown.3
Another area where there has been intensive research is the study of
sonoluminescence spectra. The spectrum for single-bubble
sonoluminescence does not appear to have any peaks that stand out, it
does not have any emission lines or spectral bands that would yield
evidence of any atomic/molecular transitions, and can easily be fit
under a blackbody curve. Under these measurements, the temperature
could be measured out to be around 16,000 Kelvin when the temperature
of the water in the experiment is at room temperature. The
temperature measured will increase to about 30,000 Kelvin when the temperature
of the water is lowered.2
Due to the nonlinear nature of the sonoluminescent bubble, there are
many theoretical research put into it by using an analytical approach.
This is due to the difficulty of experimental research that can be put
in due to conditions of the bubble and its size. In order to
accomplish an analytical approach, one must look into an equation of
motion for the surface, an equation for the energy of the liquid used,
an equation for the energy of the gas used, and a calculation on the
conservation of momentum.2
Adiabatic heating and the theory of shock waves are not the only ideas
that have been used to explain this phenomenon include chemical
reactions, plasma, Bremsstrahlung radiation, and so on, most of which
will not be mentioned in this paper.
One must say that there are many different paths that the study of
sonoluminescence yields. Nonetheless, the main attraction that
researchers have towards sonoluminescence is the incredible
concentration of energy that occurs from the collapse of the
sonoluminecent bubble. This research has brought many ideas, from
studies of acoustic cavitation to influencing chemical reactions,
The apparatus used to perform single-bubble sonoluminescence consisted
of the setup that has been used almost universally, written by
R.A. Hiller and B.P. Barber.5 Please refer to that article
for a more specific instructions.
The setup consists of two piezo-electric transducers glued with
epoxy on opposite sides of a standard 100 mL flask and a third smaller piezo-electric transducer picks up the
interaction of the sound waves and the bubble for display on the oscilloscope.
The main procedure to create single-bubble sonoluminescence is done by
trapping a bubble in the center of a spherical flask filled with .
This area is the location where the buoyancy force that allows the
bubble to rise to the surface of the flask is equal to the force
created by the resonant sound waves.3 In order to cancel
out these forces, the bubble be driven at an acoustic standing
wave.2
Equally important is the volume of the flask that is being used. The
resonant frequency used is highly dependent on the volume. The reason
the 100 mL flask was used in this experiment was because at this
volume the resonant frequency needed was about 25 kHz, a frequency
somewhat higher than human hearing. Other sized flasks could have been easily
used, but the main problem lies with people being subjected to
high-pitched hums.
In order to produce sonoluminescence, one must make the flask vibrate
at a resonant frequency. This frequency, as mentioned earlier, is
highly dependent on the size of the flask. The resonant frequency can
be theoretically be found by using the speed of sound in water (1482
m/s at 20 degrees Celsius) and dividing it by the diameter of the
spherical flask. In addition, the use of a glass flask will make the
actual frequency to be ten percent higher. Using the same
setup as everyone else, the resonant frequency comes out to be
somewhere about 25,000 Hz.5
In addition, one must first take some of the air out of the water.
This process is commonly know as degassing. There are two methods
that can be used to degas water, by boiling the water for about
fifteen minutes and the other way is by using a pump.5 The
process that was mostly used for this experiment was by using the
latter.
To create a bubble inside the flask, a simple dropper was used. First
one must extract some of the water with the dropper and then just let
a drop fall into the water in the flask. When the drop comes in
contact with the surface of the water, a couple of bubbles of air are
produced. With the function generator on around the right frequency,
the bubble of air will automatically drift towards the middle of the
flask. Now that the bubble is in place, the experimentation can
begin.
When looking at the flask for the bubble, it is convenient to be in a
dark place with a light source behind the flask. This makes the
finding of the bubble easier. Once the bubble is found, it can easily
be found at other times
In the dark, one can look at the bubble of air without looking
directly at the flask, a process that is easier to do. When the
bubble is not present, the oscilloscope will pick up the resonance of
the flask. As the bubble is introduced to the flask, the signal in
the oscilloscope shows some ripples. These shows the presence of the
bubble, showing the collapses of each cycle. Once everything is set
in order, one can then look at the flask to see the sonoluminescent
bubble.
The first experiments that were performed were done in order to get
more information about the degassing procedure. Studies were then
made by studying the behavior of the bubble. After several
measurements, an idea was developed to see how much the water needs to
be degased.
During this period sonoluminescence was sought. After a few days it
was achieved, but it was unstable (lasting about 3-5 seconds every
5-10 seconds) and the output of the light seemed to be less than it
had been reported by others.
The efforts were then directed into the improvement and the fine
tuning of the setup to increase the light intensity. Different
frequencies were measured to study the behavior of the bubble. The
graph below shows a scan of frequencies with the same degassed water
during different days.
|
 |
Frequency Range: 25,500 - 27,500 Hz Volume Range: 128 mL Input Voltage: 7.00 Volts Blue: Tuesday, 9 July 2001 Red: Wednesday, 10 July 2001 Green: Thursday, 11 July 2001
|
|
Other measurements were made to study the shape and frequency of the
acoustical resonance as a function of volume inside of the flask and
degree of degassing. The graphs below show scans of frequencies with the same
degassed water using different volumes. The procedure was repeated for three days.
|
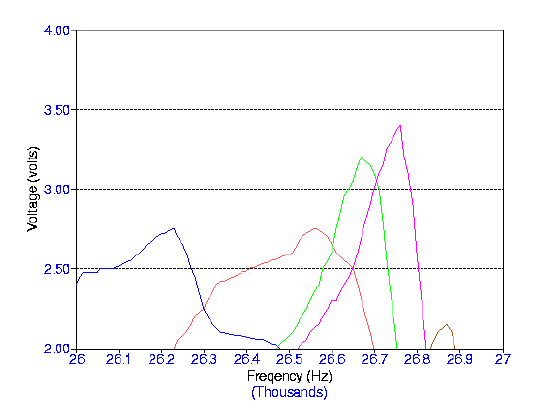 |
Frequency Range: 26,000 - 27,000 Hz Volume Range: 126 - 130 mL Input Voltage: 7.00 Volts Number of Days After Degassing: 1 Day Blue: 130 mL Red: 129 mL Green: 128 mL Violet: 127 mL Brown: 126 mL
|
|
|
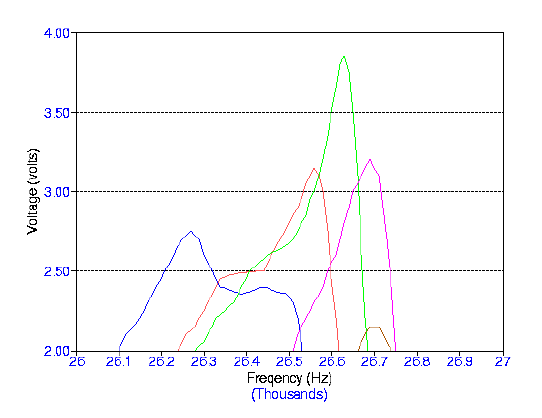 |
Frequency Range: 26,000 - 27,000 Hz Volume Range: 126 - 130 mL Input Voltage: 7.00 Volts Number of Days After Degassing: 2 Days Blue: 130 mL Red: 129 mL Green: 128 mL Violet: 127 mL Brown: 126 mL
|
|
|
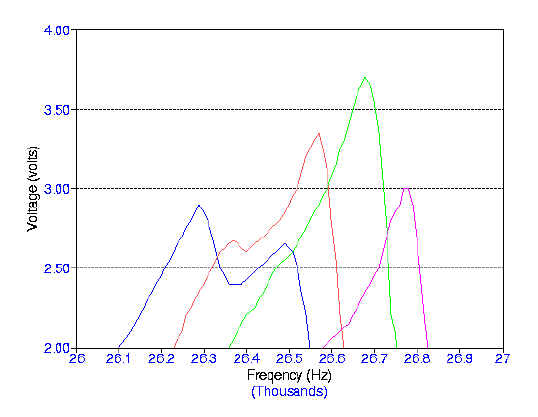 |
Frequency Range: 26,000 - 27,000 Hz Volume Range: 126 - 130 mL Input Voltage: 7.00 Volts Number of Days After Degassing: 3 Days Blue: 130 mL Red: 129 mL Green: 128 mL Violet: 127 mL Brown: 126 mL
|
|
After most of the measurements at volumes were made, our efforts were
then guided towards understanding of a more detailed approach.
Instead of making broad measurements, we concentrated on studying the
peaks.
|
|
Sonoluminescence is still a recent development in the wonderful world
of physics. There are yet many things to be studied and measured.
Only with time will one tell where sonoluminescence will lead. Only
then will there by faster and more acurate apparatus to study this
tiny bubble.
As part of the National Science Foundation program, Research
Experience for Undergraduates, the author personally feels that a lot
was learned from this experience. Due to the amount of time of the
duration of the program other experiments were not performed.
Overall, a basic understanding of sonoluminescence was achieved.
The addition of a new multimeter will defenately make some of the
measurements much easier to do. Some of the problems that were
encountered before were due to the difficulty to read measurements
using a multimeter due to the high frequencies that it is being
subjected to.
Work is also under way to observe the sonoluminescence light with a
sensitive photomultiplier tube.
The author would like to thank John Noé, Harold Metcalf, and
members of the Laser Teaching Center at State University of New York
at Stony Brook for their help, time, and patience in this experiment.
I would also like to thank Dominik Hammer and Lothar Frommhold from
the University of Texas at Austin for introducing me to
sonoluminescence when they invited Lawrence A. Crum to speak at the school.
This experiment was funded by NSF Grant No. PHY 99-12312.
1. W.C. Moss, D.B. Clarke, and D.A. Young
a. D.F. Gaitan2. L. A. Crum "Sonoluminescence" Physics Today, 22-29 (September 1994).
3. S.J. Putterman
4. H. Frenzel and H. Schultes
5. R.A. Hiller and B.P. Barber
|