Background
Carbon nanotubes are currently being proposed for numerous applications, such as, for example, catalyst supports in heterogeneous catalysis, high strength engineering fibers, sensory devices and molecular wires for the next generation of electronics devices. However, the current methods for purifying carbon nanotubes involve unreliable, low-yield processes. Also, current methods for adding oxygen moieties to carbon nanotubes are unpredictable with respect to the location of the moieties, and the types of moieties. These shortcomings of current methods present obstacles for actualizing the utility of carbon nanotubes for end-use applications. Accordingly, there remains a need for a reliable method of providing carbon nanotubes which have a high level of purity. Also, there remains a need for rationally controlling the location and types of oxygen moieties placed on carbon nanotubes.
Technology
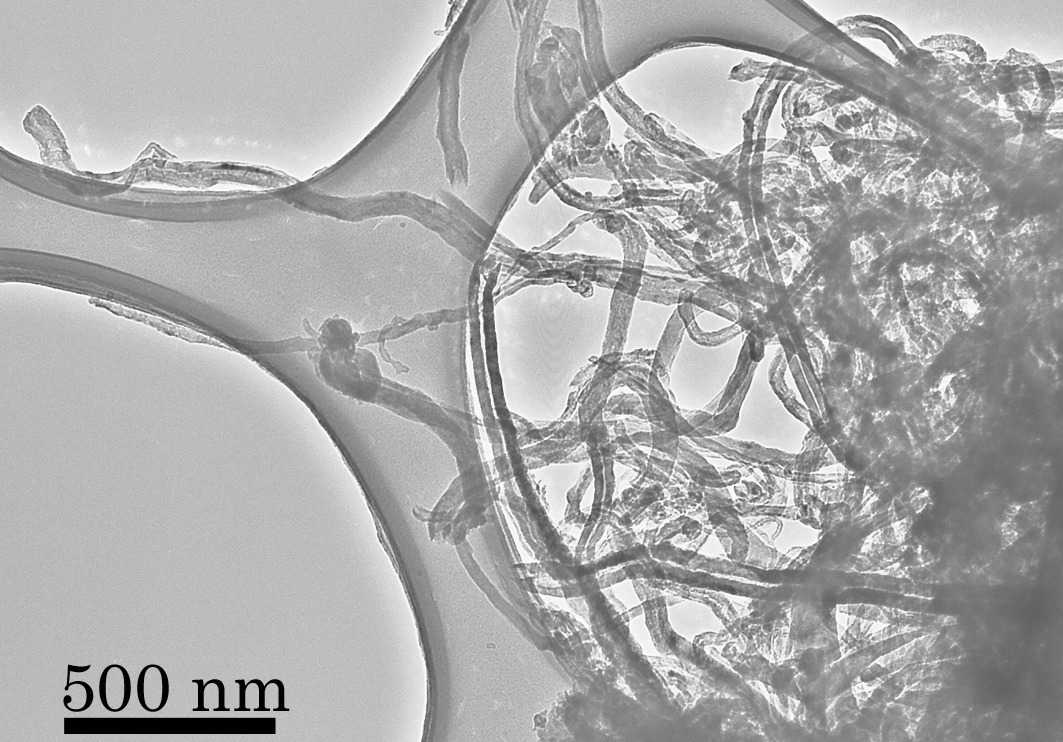
Prior studies of carbon nanotubes rely on the ability to chemically functionalize these entities in a rational and predictable manner. Ozonolysis procedures developed by researchers at Stony Brook will purify tubes of deleterious metal particles and amorphous carbon. In addition, these reactions can be used to rationally derivatize the sidewalls, as opposed to simply the ends, of nanotubes, thereby opening up a range of novel chemical applications for these structures. Moreover, the researchers have a systematic procedure to rationally skew the distribution of important oxygenated functional groups (i.e. generate high proportions of) on particular moiety through a reproducible chemical protocol, on the surfaces of the resultant purified SWNTs. That is, through this strategy, we can generate carboxylic acids, aldehydes/ketones, and alcohols on the surfaces of carbon nanotubes through chemical treatment with a variety of different standard organic reagents, that take advantage of the high reactivity of primary ozonides, that are presumed to form upon ozonolysis of SWNT dispersions in solution.
Advantages
- Novel purification procedures of raw nanotube samples - conserves structural integrity of tube bundles. - Nondestructive, low temperature chemical functionalization of nanotube sidewalls - previous derivatization procedures have focused more on derivatizing nanotubes ends. Hence, molecular species can be chemically dispersed along the sidewalls as well, with implications for the design of nanotube-based optoelectronic devices. - Tubes opened at the sides have a large potential for gas (such as hydrogen and nitrogen) storage and lithium intercalation. - Experimental demonstration of 1,3 dipolar cycloaddition to SWNT sidewalls. - Degree of control achievable over the presence of particular functional groups on the surfaces of SWNTs. Primarily ozonides will undergo oxidative cleavage with H2O2 to yield predominantly carbonyl (keto or aldehydic) functionalities. Similarly, reductive cleavage with NaBH4 leads to the generation of alcoholic groups. - Use of these derivatized SWNTs allows for linkage of these nanostructures to create novel functional composite materials
Application
- Create novel functional composite materials - With polymers, one can generate high strength materials - Attached to electrodes, electronic devices are possible - With heterogeneous catalysts, nanocatalytic applications are potentially doable - Gas storage - Lithium intercalation
Inventors
Stanislaus Wong, Professor, Chemistry
Sarbajit Banerjee, Professor, Chemistry
Licensing Potential
Licensing
Licensing Status
Available for Licensing. 7627
Licensing Contact
Donna Tumminello, Assistant Director, Intellectual Property Partners, donna.tumminello@stonybrook.edu, 6316324163
Patent Status
Patented
7,122,165 8,137,652
Tech Id
7627
